Abstract
Background and Objective : Allogeneic hematopoietic stem cell transplantation (HSCT) with myeloablative conditioning is considered the most potent post-remission antileukemic therapy for AML and MDS. Continuous evolution and improvement in conditioning regimen is a crucial step to improve the transplant outcomes and treatment-related mortality (TRM). Busulfan based regimes require constant drug monitoring and is associated with various short and long-term adverse effects, leading to exploration of less toxic regimens. Treosulfan was approved for HSCT in November 2018 in India and has been incorporated in conditioning regimens, but data on outcomes with Treosulfan based regimes is limited in South Asia. Our objective was to retrospectively analyze the emerging data in the region with Fludarabine/Treosulfan(FluTreo) and compare the outcomes with Fludarabine/Busulfan(FluBu) conditioning in AML and MDS.
Method: Consecutive patients (n=153) who underwent HSCT for AML and MDS between May 2014 and May 2022 at 5 centers with FluBu or FluTreo based conditioning were included, with Treosulphan based regimen being available only after 2018. Patients who received other conditioning protocols and those with insufficient data were excluded from the final analysis. Treosulfan 10-14gm/m2 was intravenously infused over 3 days (days -4 to -2). Busulfan 3.2mg/kg intravenously was infused over 2 days (days -4 and -3) in the comparator arm. Both groups received 30 mg/m2 intravenous Fludarabine daily for 5 days (days -6 to -2), with peripheral blood as stem cell source and Calcineurin Inhibitor (CNI) plus Methotrexate (Mtx) or Mycophenolate mofetil (MMF) in matched sibling donor as prophylaxis for graft vs host disease (GVHD). Rabbit Antithymocyte Globulin (ATG) (2.5-5 mg/kg)/ post-transplant cyclophosphamide (PTCy) were used for matched unrelated donor transplants. Haploidentical donor transplant patients received PTCy/ATG with CNI and MMF. Comorbidities were scored according to the HCT-CI. Platelet and neutrophil engraftment were defined as per standard criteria. Early toxicity after HSCT was graded according to CTCAE version 5. Acute and chronic GVHD were recorded according to standard criteria. Toxicities and outcomes between the two cohorts were compared by Chi-square test or Fisher exact test, while Overall Survival(OS) was calculated by Kaplan Meier method and the survival probabilities were compared using log-rank test. p-value < 0.05 was considered to be significant, all statistical analyses were performed using IBM SPSS Version 26 software.
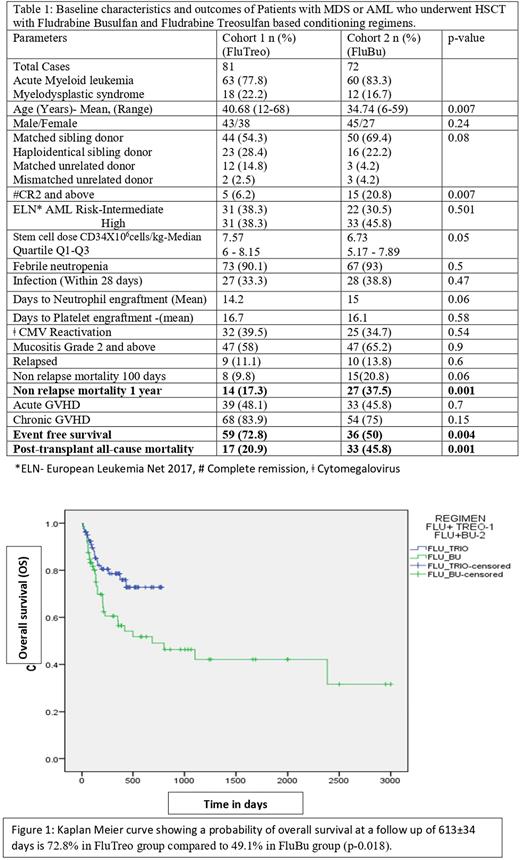
Results: Out of 153 patients, 81(52.9%) and 72(47.1%) received FluTreo and FluBu conditioning regimen respectively . FluTreo group had older patients (Mean age 40.68 years compared to 34.74 years; p-0.007) and FluBu group had more patients with second complete remission and beyond (CR2, p-0.007), but both groups were comparable on most other variables . Mucositis, infection rate, acute GVHD grade 1-4 and Chronic GVHD rates showed no significant difference between the groups(Table 1). We observed a better 1 year non relapse mortality (17.3% Vs. 37.5%, p=0.001), lesser all-cause mortality (20.9% Vs. 45.8%; p=0.001) and a better event free survival (72.8% v/s50.1% p=0.004) in FluTreo group. At a follow up of 613±34 days the Kaplan Meier curve showed an overall survival probability of 72.8% in the FluTreo group, compared to 49.1% in FluBu group (p=0.018; Figure 1).
Conclusion: FluTreo conditioning showed favorable outcomes in terms of decreased all-cause mortality and improved overall survival with comparable immediate and long-term adverse events in comparison with FluBu. However the study is limited by its retrospective design, the conditioning regimens being tried in two different time periods and the shorter follow up of the FluTreo group. Considering the difficulties in the feasibility of conducting a prospective randomized control trial in this setting, we believe our study shows early insights into safety and improved efficacy of FluTreo conditioning regimen in AML and MDS .
Disclosures
Sidharthan:Astra Zeneca: Speakers Bureau; Emcure: Consultancy; Takeda: Speakers Bureau; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Roche: Membership on an entity's Board of Directors or advisory committees; jansen: Speakers Bureau; Novo Nordisk: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal